What is Ebstein anomaly?
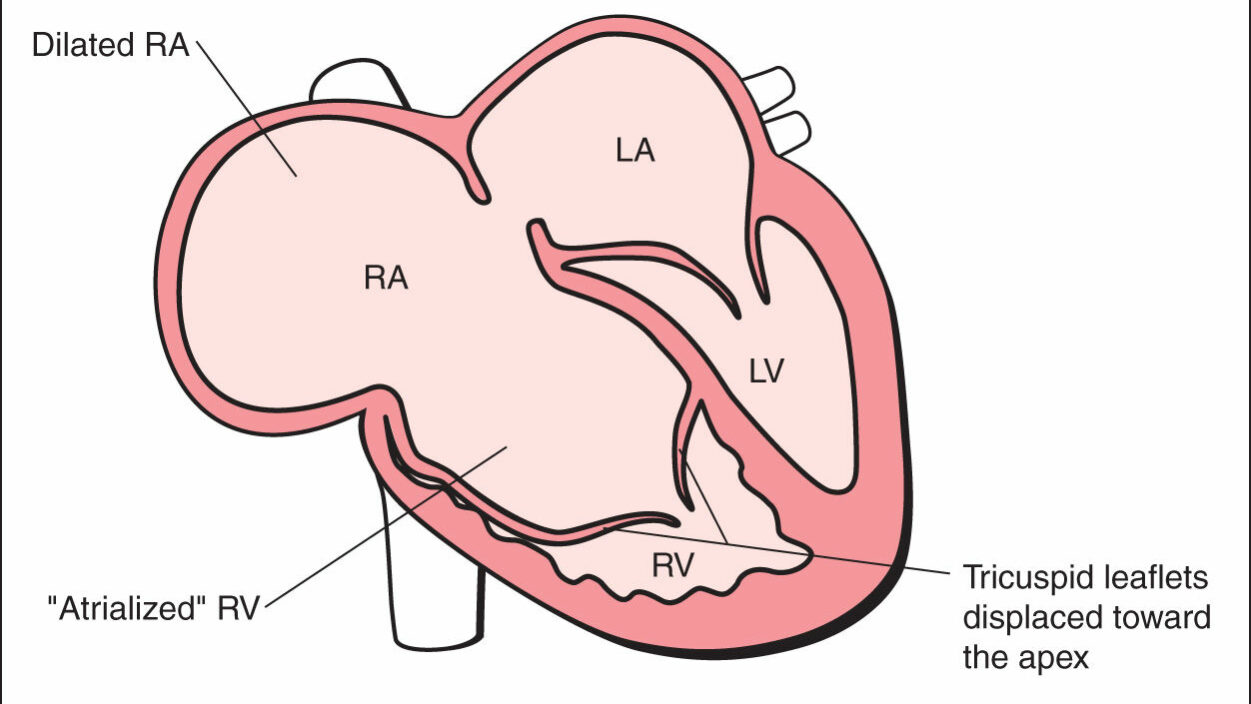
Ebstein’s anomaly is a rare congenital heart defect affecting the tricuspid valve, which separates the right atrium (right collecting chamber) from the right ventricle (right pumping chamber). It occurs in approximately 1 in 20,000 live births. Anatomical Features: In Ebstein’s anomaly, the tricuspid valve leaflets are displaced downward into the right ventricle, rather than sitting at the normal atrioventricular junction. This creates:
- An enlarged right atrium
- A smaller functional right ventricle (the “atrialized” portion becomes part of the right atrium)
- Tricuspid valve regurgitation (leakage)
Symptoms of Ebstein anomaly
The symptoms of Ebstein’s anomaly vary significantly depending on the severity of the valve displacement and associated defects. Some patients may have no symptoms, while others experience significant cardiovascular compromise.
Newborns and Infants:
- Cyanosis (blue discolouration of lips, skin, or nail beds)
- Tachypnea (rapid breathing) and difficulty feeding
- Poor weight gain and failure to thrive
- Heart murmur detected on examination
- Signs of heart failure in severe cases
Children and Adolescents:
- Exercise intolerance and fatigue with physical activity
- Shortness of breath during exertion or at rest in severe cases
- Palpitations or awareness of irregular heartbeat
- Chest pain (less common)
- Syncope (fainting) or presyncope (near-fainting)
- Intermittent cyanosis, particularly with crying or exertion
Adults:
- Progressive exercise limitation as the condition may worsen over time
- Atrial arrhythmias, particularly atrial fibrillation or flutter
- Supraventricular tachycardia (especially if Wolff-Parkinson-White syndrome is present)
- Right heart failure symptoms: ankle swelling, abdominal distension
- Paradoxical embolism (rare but serious complication)
Severity Classifications:
- Mild: Often asymptomatic with normal exercise capacity
- Moderate: Symptoms typically appear during childhood or adolescence
- Severe: Usually presents in infancy with significant symptoms
Associated Complications:
- Arrhythmias occur in up to 25% of patients
- Stroke risk due to right-to-left shunting and arrhythmias
- Infective endocarditis risk (antibiotic prophylaxis may be recommended)
Causes of Ebstein anomaly
Ebstein’s anomaly is a congenital heart defect that develops during fetal cardiac development, typically between the 6th and 10th weeks of pregnancy when the tricuspid valve is forming.
Developmental Mechanism: The condition occurs when the tricuspid valve leaflets fail to separate properly from the underlying heart muscle (myocardium) during embryonic development. Normally, the valve leaflets should delaminate from the ventricular wall and move to their correct position at the atrioventricular junction.
Known Risk Factors:
Maternal Medications:
- Lithium exposure during pregnancy is the most well-established risk factor
- Risk increases 10-20 fold with first-trimester lithium use
- Benzodiazepines have also been associated with increased risk
- Some anticonvulsants may contribute to risk
Maternal Conditions:
- Diabetes mellitus (poorly controlled)
- Maternal age (some studies suggest slightly increased risk with advanced maternal age)
- Maternal infections during early pregnancy (though less well established)
Genetic Factors:
- Most cases are sporadic (occur randomly)
- Familial cases are rare but do occur, suggesting possible genetic components
- Associated with some genetic syndromes though this is uncommon
- Chromosomal abnormalities are occasionally present
Environmental Factors:
- Alcohol consumption during pregnancy may contribute to risk
- Smoking during pregnancy has been suggested as a possible risk factor
- Nutritional deficiencies (particularly folate) may play a role
Important Points:
- In the vast majority of cases, no specific cause can be identified
- Most mothers of babies with Ebstein’s anomaly have no identifiable risk factors
- The condition is not preventable in most instances
- Prenatal diagnosis is possible with fetal echocardiography, usually after 18-20 weeks of pregnancy
Counselling for Families: It’s crucial to emphasise to parents that in most cases, nothing they did or didn’t do caused their child’s condition. Ebstein’s anomaly typically results from random developmental events during early pregnancy, and parents should not feel responsible for their child’s diagnosis.
Genetic Counselling: While most cases are sporadic, families may benefit from genetic counselling if there’s a family history of congenital heart disease or if considering future pregnancies.
Diagnosis of Ebstein’s anomaly
The diagnosis of Ebstein’s anomaly involves a combination of clinical assessment and specialized cardiac imaging. Early and accurate diagnosis is crucial for determining appropriate management strategies.
Clinical Assessment:
Physical Examination Findings:
- Heart murmur: Typically a systolic murmur due to tricuspid regurgitation
- Cyanosis: May be present, particularly in severe cases or with exertion
- Jugular venous distension: In cases with significant tricuspid regurgitation
- Hepatomegaly: Due to right heart failure in severe cases
- Clubbing: May develop in patients with chronic cyanosis
- Gallop rhythm: Third or fourth heart sounds may be audible
Diagnostic Investigations:
Echocardiography (Primary Diagnostic Tool):
- Transthoracic echocardiography is the gold standard for diagnosis
- Key measurements: Displacement index (>8mm/m² body surface area is diagnostic)
- Assessment of valve morphology: Degree of leaflet tethering and mobility
- Quantification of regurgitation: Severity of tricuspid valve leak
- Right heart dimensions: Size of right atrium and functional right ventricle
- Associated defects: Detection of atrial septal defects or patent foramen ovale
- Fetal echocardiography: Can diagnose condition prenatally after 18-20 weeks
Electrocardiogram (ECG):
- Right bundle branch block: Present in 85-90% of patients
- First-degree AV block: Prolonged PR interval
- Right atrial enlargement: Tall P waves in lead II
- Pre-excitation patterns: Delta waves if Wolff-Parkinson-White syndrome present
- Atrial arrhythmias: May show atrial fibrillation or flutter
Chest X-ray:
- Cardiomegaly: Enlarged cardiac silhouette, particularly right heart
- Reduced pulmonary vascular markings: In cases with reduced pulmonary blood flow
- “Box-shaped heart”: Classic appearance due to enlarged right atrium
Advanced Imaging:
Cardiac MRI:
- Detailed anatomical assessment: Superior tissue characterization
- Functional evaluation: Precise measurement of ventricular volumes and function
- Planning for surgery: Detailed pre-operative assessment
- Long-term follow-up: Monitoring of right ventricular function
Cardiac Catheterization:
- Rarely required for diagnosis in the current era
- May be indicated for assessment of pulmonary vascular resistance before surgery
- Electrophysiology study: If significant arrhythmias are present
Severity Classification:
Carpentier Classification (Anatomical):
- Type A: Mild displacement with good leaflet mobility
- Type B: Large anterior leaflet with restricted motion
- Type C: Severe displacement with poor leaflet development
- Type D: Almost complete absence of tricuspid valve tissue
Functional Assessment:
- Great Ormond Street Score: Combines anatomical and functional parameters
- Celermajer Index: Relates right atrial area to combined left heart area
- Tricuspid regurgitation severity: Mild, moderate, severe, or torrential
Differential Diagnosis:
- Tricuspid atresia: Complete absence of tricuspid valve
- Tricuspid stenosis: Narrowed but normally positioned valve
- Uhl’s anomaly: Absent right ventricular myocardium
- Arrhythmogenic right ventricular cardiomyopathy: Acquired condition with replacement of some of the tissue of the muscle tissue in the right ventricle with scar/fat tissue.
Timing of Diagnosis:
- Prenatal: Fetal echocardiography can detect severe cases
- Neonatal: Severe cases typically diagnosed in first days/weeks of life
- Childhood/Adolescence: Moderate cases may present during routine examinations
- Adulthood: Mild cases occasionally diagnosed during investigation of symptoms
Follow-up Monitoring: Regular echocardiographic surveillance is essential to monitor:
- Progression of tricuspid regurgitation
- Right heart dimensions and function
- Development of arrhythmias
- Need for surgical intervention
The key to successful management is early recognition and establishment of appropriate long-term cardiology follow-up, regardless of initial symptom severity.
Treatment of Ebstein anomaly
Treatment of Ebstein’s anomaly is highly individualized and depends on the severity of the anatomical defect, clinical symptoms, functional status, and associated complications. Management ranges from observation to complex surgical intervention, with the approach tailored to each patient’s unique circumstances.
Conservative Management
For many patients with Ebstein’s anomaly, particularly those who are asymptomatic or have mild symptoms, conservative management forms the cornerstone of care. This approach involves regular cardiology follow-up with annual or biannual appointments, allowing for careful monitoring of the condition’s progression over time. Serial echocardiography provides detailed assessment of valve function and right heart dimensions, while ECG monitoring helps detect the development of arrhythmias, which are common in this population. Exercise testing plays a crucial role in assessing functional capacity and guiding activity recommendations, with most patients able to participate in normal daily activities and even many sports. Endocarditis prophylaxis may be recommended for certain dental or surgical procedures, particularly in patients with complex anatomy or previous surgical interventions.
Medical Management
When symptoms develop or complications arise, medical therapy becomes an important component of treatment. Heart failure, when present, is typically managed with ACE (Angiotensin-converting enzyme) inhibitors or ARBs (Angiotensin receptor blockers) to reduce afterload on the right ventricle, while diuretics help manage volume overload and symptoms of right heart failure. Digoxin may provide benefit in some patients with heart failure, though its use requires careful monitoring. Beta-blockers are used cautiously in this population but can be helpful for managing arrhythmias when appropriate.
Arrhythmia management represents a significant aspect of care for many patients with Ebstein’s anomaly. Antiarrhythmic medications such as flecainide, sotalol, or amiodarone may be necessary for controlling symptomatic arrhythmias. For patients who develop atrial fibrillation or flutter, rate control strategies are implemented alongside consideration for anticoagulation, particularly in those with associated right-to-left shunting who face increased thromboembolic risk.
Interventional Procedures
Catheter-based interventions play an increasingly important role in managing certain aspects of Ebstein’s anomaly. Radiofrequency ablation is particularly valuable for patients with Wolff-Parkinson-White syndrome, which occurs in approximately 10-25% of individuals with this condition. When anatomically suitable, atrial septal defects can be closed using transcatheter percutaneous occluder devices, though this approach requires careful evaluation to ensure it won’t compromise cardiac output. Transcatheter balloon valvuloplasty has limited applicability due to the complex morphology of the tricuspid valve in Ebstein’s anomaly.
Surgical Treatment
The decision to proceed with surgical intervention requires careful consideration of multiple factors. Surgery is typically recommended for patients with progressive symptoms despite optimal medical therapy, significant functional limitation, progressive right heart enlargement or dysfunction, severe symptomatic tricuspid regurgitation, paradoxical embolism, or intractable arrhythmias. The timing of surgical intervention is crucial, as operating too early may subject patients to unnecessary risk, while delaying surgery too long may compromise outcomes.
Tricuspid valve repair is the preferred surgical approach whenever technically feasible. The cone reconstruction technique has emerged as the current gold standard, offering excellent outcomes in appropriately selected patients. This technique involves creating a cone-shaped valve apparatus from the existing leaflet tissue, providing more competent valve function. The Carpentier technique remains valuable for patients with suitable anatomy, while the Danielson technique is used less frequently in contemporary practice. Concurrent procedures often include closure of atrial septal communications and right atrial reduction when the atrium is significantly enlarged.
When valve repair is not feasible due to severe anatomical distortion or inadequate leaflet tissue, tricuspid valve replacement becomes necessary. Bioprosthetic valves are generally preferred, particularly in younger patients, as they avoid the need for lifelong anticoagulation. Mechanical valves, while durable, require lifelong warfarin therapy and carry associated bleeding risks. Valve replacement procedures generally carry higher operative risk compared to repair procedures.
For patients with the most severe forms of Ebstein’s anomaly, particularly those with inadequate biventricular circulation, more complex surgical strategies may be required. These can include bidirectional cavopulmonary shunt procedures or, in extreme cases, Fontan completion to establish single ventricle physiology. Heart transplantation remains a last resort for patients with end-stage disease who are not candidates for other interventions.
Neonatal Management
Newborns presenting with severe Ebstein’s anomaly represent a particularly challenging population. These infants may require prostaglandin E1 infusion to maintain ductal patency and ensure adequate systemic oxygenation. Mechanical ventilation and inotropic support may be necessary to stabilize critically ill neonates. In some cases, urgent surgical intervention may be required, potentially including systemic-to-pulmonary shunts to improve oxygenation in the setting of severe right heart dysfunction.
Pregnancy Considerations
Women with Ebstein’s anomaly require specialized care when considering pregnancy. Pre-pregnancy counseling should include comprehensive risk assessment based on functional status and anatomical severity, genetic counseling regarding recurrence risk in offspring, optimization of medical therapy, and discussion of appropriate contraceptive options. During pregnancy, these patients require high-risk obstetric care with close cardiology involvement, regular monitoring of cardiac function throughout gestation, careful anticoagulation management when indicated, and planned delivery in a tertiary center with appropriate expertise.
Long-term Follow-up and Outcomes
All patients with Ebstein’s anomaly require lifelong cardiology care, with the frequency of follow-up determined by the severity of their condition and functional status. Serial imaging is essential to monitor right heart function over time, while arrhythmia surveillance, including periodic Holter monitoring, helps detect the development of rhythm disturbances. Exercise testing guides activity recommendations and helps assess the need for intervention. As patients transition from pediatric to adult care, coordination with adult congenital heart disease services ensures continuity of specialized care.
The outcomes for patients with Ebstein’s anomaly have improved significantly with advances in surgical techniques and perioperative care. Contemporary tricuspid valve repair procedures achieve 85-95% freedom from reoperation at 10 years, with early mortality rates of 2-5% for elective procedures. Most patients experience significant symptom relief and excellent quality of life following successful repair. The long-term prognosis varies considerably based on the severity of disease, with patients having mild disease enjoying near-normal life expectancy, while those with severe disease require ongoing complex management but benefit from improving outcomes with modern treatment approaches.
The fundamental principle underlying all treatment decisions is that each patient’s care must be individualized based on their specific anatomy, physiology, and clinical circumstances. The timing of intervention requires careful consideration, as premature or delayed treatment can compromise outcomes. A multidisciplinary team approach, involving pediatric and adult congenital cardiologists, cardiac surgeons, and electrophysiologists, ensures comprehensive care. Throughout this process, patient and family education remains essential for achieving optimal long-term outcomes and ensuring patients can make informed decisions about their care. The ultimate goal of treatment is to optimize functional capacity, prevent complications, and ensure the best possible quality of life throughout the patient’s lifetime.
Cone repair for Ebstein’anomaly
The cone reconstruction technique represents a revolutionary approach to surgical repair of Ebstein’s anomaly, pioneered by Dr. José Pedro da Silva and further developed at institutions like the Mayo Clinic. This technique has fundamentally changed the surgical management of this complex congenital heart defect and has become the current gold standard for tricuspid valve repair in Ebstein’s anomaly.
Surgical Technique and Principles
The cone repair technique is based on the principle of utilizing all available tricuspid valve leaflet tissue to create a functional valve. The cone repair results in 360° of tricuspid leaflet tissue surrounding the right atrioventricular junction, which distinguishes it from earlier repair techniques that often relied on anterior leaflet monocusp methods.
The procedure involves complete mobilization of the tricuspid valve leaflets from their abnormal attachments to the right ventricular wall. The leaflets are then rotated and sutured together to create a cone-shaped valve apparatus that sits at the proper anatomical location of the tricuspid annulus. This approach maximizes the use of native valve tissue while restoring normal valve geometry and function.
The technical aspects of the procedure require meticulous attention to detail, as the success of the repair depends on proper leaflet mobilization, appropriate sizing of the valve orifice, and secure closure of the atrialized portion of the right ventricle. Concurrent procedures often include closure of associated atrial septal defects and plication of the enlarged right atrium.
Clinical Outcomes and Effectiveness
The cone reconstruction technique has demonstrated excellent clinical outcomes across multiple studies. This technique was performed in 100 patients with a hospital mortality rate of 3.0%, good clinical outcome, and no need for tricuspid valve replacement. These results represent a significant improvement over earlier surgical techniques and highlight the safety and effectiveness of the procedure.
Da Silva’s cone repair for Ebstein’s anomaly creates excellent valve function in all patients. Consecutively, the size of the RV decreases and the antegrade net stroke volume increases 6 months after the operation. This demonstrates not only the immediate functional improvement but also the positive remodeling effects on right ventricular function that occur following successful repair.
Early postoperative echocardiograms have shown good right ventricular morphology and reduction in tricuspid regurgitation grade from 3.6 +/- 0.5 to 1.2 +/- 0.5 (P < .0001), indicating substantial improvement in valve competency. The functional benefits translate into improved exercise capacity and quality of life for patients.
Long-term Durability and Follow-up
One of the key advantages of the cone repair is its durability. Mayo Clinic heart surgeons pioneered the cone reconstruction technique to correct Ebstein anomaly. Outcome data shows the technique is safe, durable and effective. Long-term follow-up studies have shown excellent freedom from reoperation, with most patients maintaining good valve function years after surgery.
However, questions remain about the long-term durability in certain patient populations, particularly pediatric patients. However, in their study, patients were older and the follow-up duration was not long enough to answer the questions regarding the effect of cone reconstruction and its durability in younger age groups, indicating the need for continued long-term surveillance.
Right Ventricular Remodeling
One of the remarkable aspects of the cone repair is its effect on right ventricular remodeling. Following successful cone reconstruction, patients typically experience normalization of right heart dimensions and improvement in ventricular function. The reduction in tricuspid regurgitation leads to decreased volume loading of the right ventricle, allowing for reverse remodeling and improved cardiac efficiency.
This remodeling process continues over months following surgery, with progressive improvement in right ventricular size and function. The restoration of more normal tricuspid valve function also improves the efficiency of right ventricular filling and emptying, contributing to better overall cardiac performance.
Learning Curve and Technical Considerations
Increasing familiarity with Ebstein’s anomaly and Cone reconstruction led to a reduction in resource utilization, highlighting the importance of surgical experience and institutional expertise. The cone repair is a technically demanding procedure that requires considerable expertise in congenital heart surgery and a thorough understanding of the complex anatomy of Ebstein’s anomaly.
The procedure requires careful preoperative planning using detailed echocardiographic and sometimes cardiac MRI imaging to understand the specific anatomy and plan the optimal surgical approach. Intraoperative decision-making is crucial, as the extent of leaflet tissue available for reconstruction varies considerably between patients.
Patient Selection and Contraindications
The cone repair is applicable to most patients with Ebstein’s anomaly who have adequate leaflet tissue for reconstruction. The technique can be successfully applied across a wide age range, from infants to adults, though the specific technical approach may need modification based on patient size and anatomical variants. Contraindications to cone repair are relatively few but may include cases with severely dysplastic or absent leaflet tissue, extensive right ventricular dysfunction that precludes biventricular repair, or concurrent conditions that significantly increase operative risk.
Comparison with Other Techniques
The cone repair has largely replaced earlier techniques such as the Carpentier and Danielson repairs due to its superior outcomes and durability. Unlike these earlier methods, which often involved significant reduction of the tricuspid valve orifice area, the cone technique preserves or even increases the effective valve area while maintaining competency.
The procedure represents a significant advancement in the surgical treatment of Ebstein’s anomaly, offering patients the best opportunity for long-term freedom from tricuspid regurgitation and preservation of biventricular function. Its success has made it the preferred technique at most major congenital heart surgery centers worldwide.