Tetralogy of Fallot: symptoms, causes, treatment and repair
Tetralogy of Fallot is a condition where there is a narrowing in the blood flow from the right ventricle to the lungs, as a consequence of thick muscle underneath the pulmonary valve and a small pulmonary valve. There is also a VSD (hole between the pumping chambers) which causes the oxygen level in the blood to drop.
What is Tetralogy of Fallot?
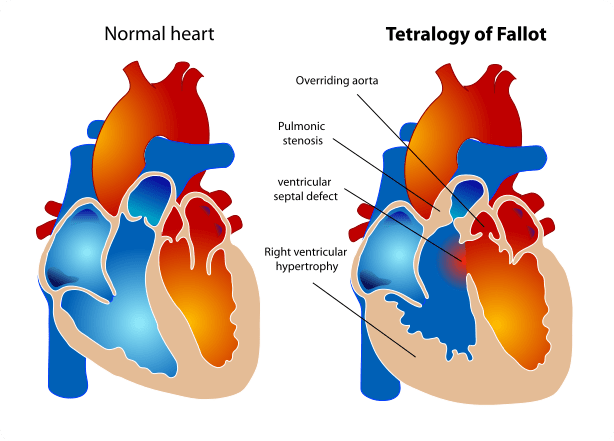
Tetralogy of Fallot is a heart defect that features four main problems. These problems are:
- a hole between the lower chambers of the heart: the pumping chambers, also called the ventricles. This hole is called a ventricular septal defect or VSD;
- an obstruction to the blood flow from the heart to the lungs called the pulmonary stenosis;
- the main body artery, called the aorta, lies over the hole in the lower chambers (overrides the VSD);
- the muscle surrounding the lower right chamber becomes overly thickened Tetralogy of Fallot is a relatively frequent type of congenital cardiac defects and is present in 1 in 3000 live births.
What causes Tetralogy of Fallot?
In the majority of children, the cause of tetralogy of Fallot isn’t known or is not obvious. Tetralogy of Fallot is a common type of congenital heart defect. Tetralogy of Fallot can be seen more commonly in children with genetic syndromes like Di George syndrome or Down syndrome (trisomy of chromosome 21).
Some children may have some other heart defects along with tetralogy of Fallot. An example of this is complete AV septal defect associated with Down’s syndrome.
Even though tetralogy of Fallot is not strictly hereditary, in some families there can be several family members affected by the same condition or different congenital heart defects, suggesting there is some genetic influence.
How does Tetralogy of Fallot affect the heart and blood flow?
Normally the left sided pumping chamber of the heart, the left ventricle, only pumps blood to the body, through the main body artery called the aorta.
The heart’s right sided pumping chamber, the right ventricle, only pumps blood to the lungs.
In a child with tetralogy of Fallot, low oxygen blood can travel across the hole between the 2 pumping chambers (VSD) from the right pumping chamber (right ventricle) to the left pumping chamber (the left ventricle) and from there the blood will flow out into the main body artery (the aorta).
This causes low oxygen blood which should have gone to the lungs to bypass the lungs and go in the main body circulation which should only contain highly oxygenated blood, as shown in the figure above. The presence of obstruction in the flow of blood across the pulmonary valve (which connects the right ventricle to the lung arteries) prevents a normal flow of low oxygenated blood from being pumped to the lungs. In some of the most extremes cases, like when the pulmonary valve is completely obstructed (pulmonary atresia), the whole of low oxygenated blood has to stream to the main body circulation trough the VSD.
Is tetralogy of Fallot the same in every child?
There is a considerable variation in the heart appearance in children with tetralogy of Fallot and this can greatly influence the symptoms, the clinical presentation and the timing of diagnosis, the timing of surgery and the type of surgery that is required. For example some children with small connection between the right-sided pumping chamber, the right ventricle, and the pulmonary artery or those with small pulmonary arteries might develop a blue tinge due to low oxygen level, called cyanosis, soon after birth.
These children may require initial treatment in the first few days of life. Some other children may have very little obstruction to the flow of blood to the lungs and they may never develop cyanosis but would rather show signs of breathlessness and may struggle to feed and put on weight.
A paediatric cardiologist needs to examine many details of the make-up of the heart in order to plan safe and effective surgical treatment.
How does tetralogy of Fallot affect my child?
Infants and young children with unrepaired tetralogy of Fallot are often blue, cyanotic.
In some children tetralogy of Fallot may have been diagnosed antenatally before birth.
This is however the case form the minority of children. A child may be referred to see a paediatric cardiologist for a variety of reasons and at different ages. Some newborns with very significant restriction to the blood flow to the lungs will present in the first hours/days of life with severe cyanosis and unable to feed or looking very sleepy.
The reason for the cyanosis is that some of the oxygen-poor blood is pumped to the body through the VSD instead of being pumped to the lungs. In some instances a child might be referred for the finding of cyanosis, blue appearance of the skin, and lips/finger tips, whereas other times they may be referred for signs of excessive blood to the lungs (fast breathing, poor feeding, poor weight gain typical of pink tetralogy where the obstruction to the flow to the lungs is minimal or absent). Some children may be referred because of a murmur is picked up by the general practitioner or by the paediatrician.
How is tetralogy of Fallot diagnosed?
Tetralogy of Fallot may be suspected in children with cyanosis and a heart murmur, a noise produced by the fast blood flow through the heart. A detailed diagnosis is established by echocardiography, ultrasound scan of the heart. This technique allows to define all the details that are required for the appropriate management of the condition. In a minority of children, usually those with small pulmonary arteries, those who have had previous palliative procedures (see below), and in older children and adolescents, some more imaging may be required to define the most appropriate treatment plan. These tests usually include a computed tomography (CT) scan of the heart, an MRI scan of the heart or a cardiac catheterisation and angiography.
What can be done about tetralogy of Fallot?
Complete repair
The treatment of tetralogy of fallout is surgical. The optimal age for complete repair of isolated tetralogy of Fallot is between 3 and 6 months, even though an earlier age has been recently advocated by some centres.
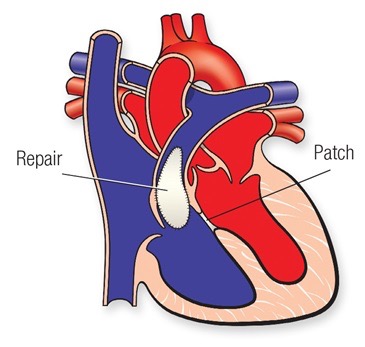
Complete repair requires the closure of the hole between the 2 pumping chambers, the VSD, usually achieved with the use of a patch, and enlargement of the area connecting the right ventricle to the pulmonary artery (called the right ventricular outflow tract or RVOT).
This can be achieved in different ways depending on where the narrowing is located.
In some children, where the narrowing is mainly due to thick muscle underneath the pulmonary valve, muscle resection in isolation or associated with a patch enlargement might be required.In children where the narrowing is mainly across the pulmonary valve, the patch might need to be extended through the valve, trans-annular patch. In some other children, where the main lung artery distal to the valve is narrow or the narrowing extends to the right or the left pulmonary artery, a patch might need to be applied to enlarge those areas.
In some children the surgeon needs to use a tube, with or without a valve inside, to connect the right sided pumping chamber to the pulmonary artery.
This is known as the Rastelli operation.
Temporary (palliative) procedures
These include:
-
Use of prostaglandines (PGE): some small children with very limited amount of blood flow may need to be started on intra-venous infusion (a drip) of prostaglandins in order to keep the arterial duct open.
-
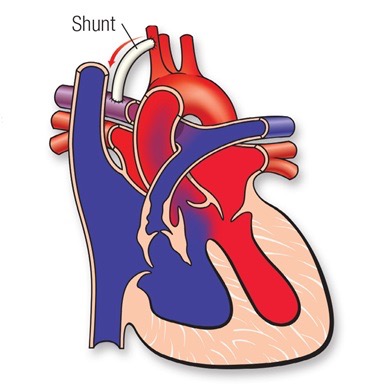
Creation if an additional source of blood flow with a shunt operation. This operation (shown above) is undertaken to provide an adequate blood flow to the lungs. This procedure is not open-heart surgery and does not fix the problems on the inside of the heart. The shunt consists in a small tube of Goretex (usually 3.5 to 4.0 mm in diameter) placed between an upper body artery (or the aorta) and the pulmonary artery (the right pulmonary artery usually). The shunt is closed and taken down at the time of complete repair.
-
Creation of an additional source of blood flow by using a conduit (a goretex tube or other suitable conduit) between the right ventricle and the pulmonary artery. This procedure is undertaken in children where there are anomalies of the coronary arteries (the arteries that feed the heart itself). Because of the anomaly in the position of the coronary arteries, a usual complete repair cannot be undertaken in order not to damage the coronary arteries. The use of a conduit in a
-
non anatomical position allows to increase the blood flow to the lungs avoiding damage to the coronary arteries
-
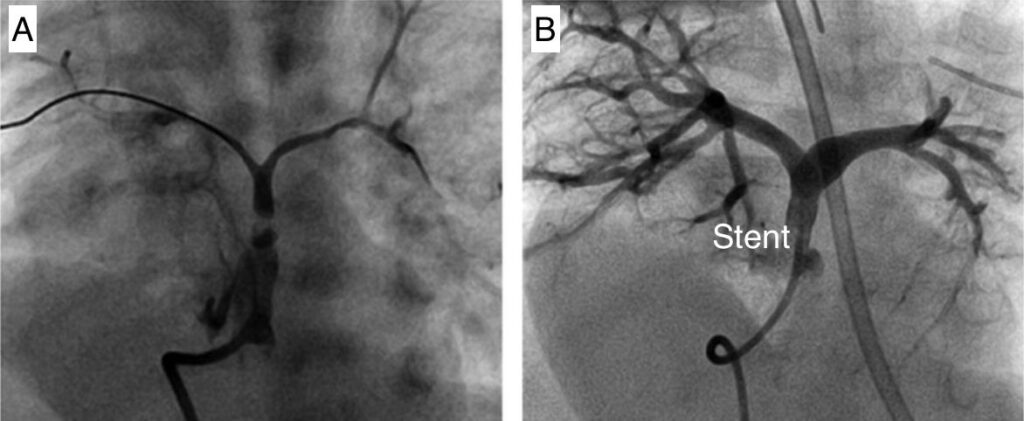
implantation of a stent in the narrow area between the right ventricle and the pulmonary artery. This highly skilled procedure can be undertaken as a key-hole procedure and does not required any cut on the patient chest. This procedure can be useful in some small children with narrow connections between the right ventricle and the pulmonary arteries and consists in the placement of a stent across the narrow area with the aim of improving the blood flow through the lungs. This procedure is typically used in newborns who present with low oxygen saturations and even though it allows oxygen to improve and the child to grow. It is only a way of stabilizing the child as a complete repair is still going to be required as a later stage. The image below shows an injection of dye (angiogram) before (A) and after the placement of a stent (B). The image on the right shows much more blood and dye going to the lung arteries with much improved oxygen level.
-
Repair of tetralogy of Fallot: surgery success rate, recovery time, and complications
The success rate for repair of tetralogy of Fallot is 99% so the procedure is very effective and safe. Children who undergo repair of tetralogy of Fallot without complications usually require to spend 7-10 days in hospital after surgery. The advice is to refrain from school attendance and vaccinations for 4 weeks. It may take longer after surgery to be able to resume physical activity.
Post-operative complications of tetralogy of Fallot are uncommon and the most common are: bleeding from the surgery site, infections and problems with the heart rhythm.
Will my child’s activities be limited?
The majority of children with fully repaired tetralogy of Fallot will have a normal quality of life including the ability to play sports and to attend mainstream school.
Many children and young adults with tetralogy of Fallot can take part in competitive sport but very intense sport participation may be a problem for some. Specific guidelines and advice on sport participation are available even though the child’s paediatric cardiologist in consultation with the child and family should be the one providing individualized advice. Shaun White, the 3 Olympic gold medals winner for snowboarding, was born with tetralogy of Fallot and has received full repair and has been an inspiration to young people with the same condition.
What will my child need in the future?
Every child with tetralogy of Fallot will require life-long follow-up, even in adult age. Most children and young adults (those without significant narrowing or leakage in the pulmonary valve, those with normal heart function, and those with no rhythm problems) will require to see their cardiologists every 1-2 years and will required ECGs and echocardiograms on those occasions. Some patients will require to undertake cardiac MRI scans and exercise test every few years as part of the disease surveillance. Those patients with residual problems might need to see their cardiologist more often or may require additional tests of procedures.
What is the life expectancy of someone with tetralogy of Fallot?
It is difficult to predict how long a child with repaired Tetralogy of Fallot is going to live but data suggests that the outcome is generally good up to 30-40 years after complete repair. We do not much beyond this point.
Knowledge about this topic is made even more difficult as recent advances in the surgical techniques and intensive care management will have had a positive effect on survival rates early and late after surgery. Some adults will require further surgery later in life as a result of a leaking pulmonary valve. Exercise capacity will be reduced in some adults with repaired tetralogy of Fallot.